概要
最終目標を見据えた抗体開発
溶解性、安定性、凝集性など、開発要件に関わるパラメーターは有望なリード抗体を決めるための重要な要素です。
Twist Biopharma Solutions では、抗体発現、評価を含めた幅広い開発要件評価パッケージを提供しています。保有のクロマトグラフィー、分光光度計、蛍光測定機器群を使いて、開発工程、製剤化工程を見据えた最適な抗体候補を選ぶための柔軟な評価プログラムを提供します。
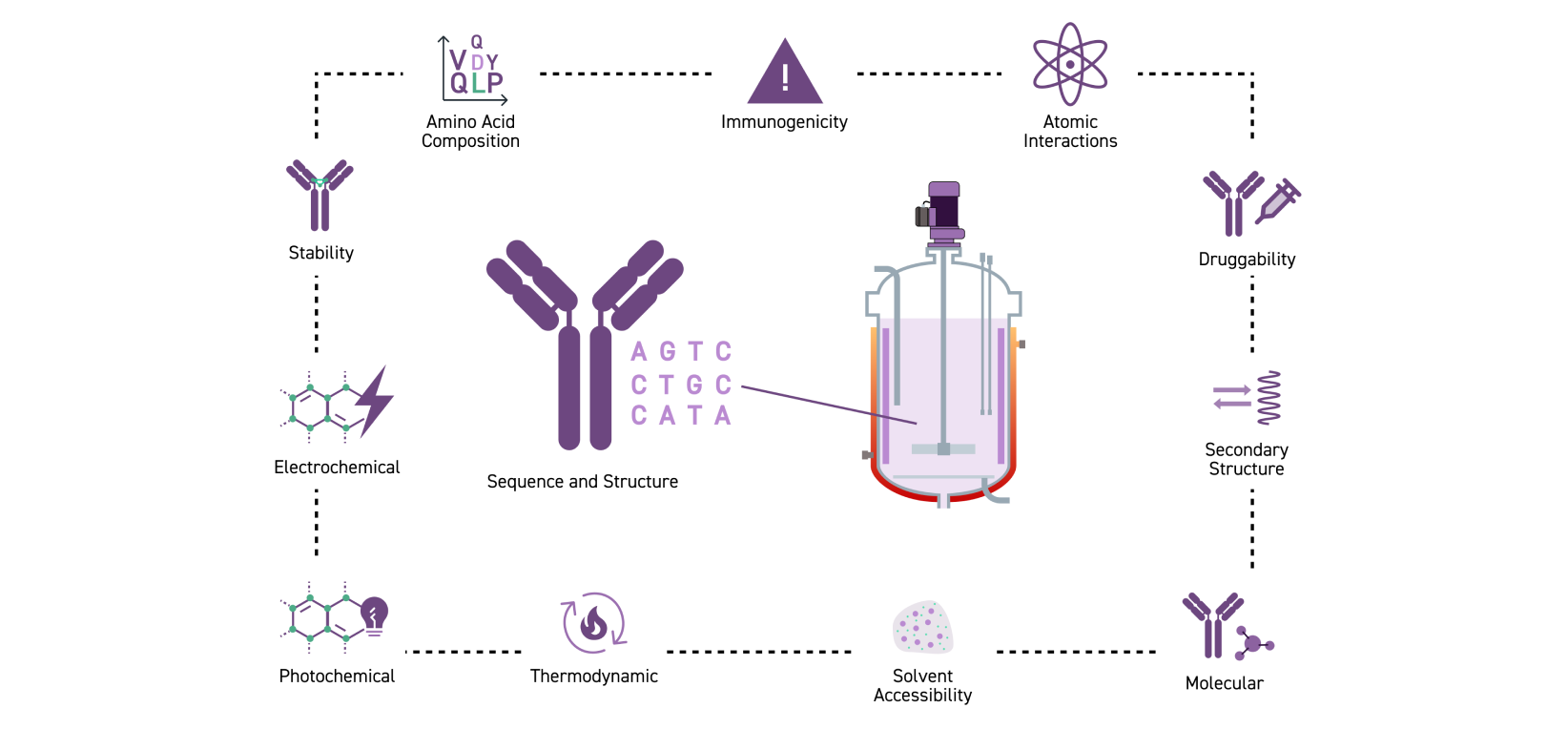
柔軟な開発可能性評価パッケージ
最終目標を見据えた抗体開発
溶解性、安定性、凝集性など、開発要件に関わるパラメーターは有望なリード抗体を決めるための重要な要素です。
Twist Biopharma Solutions では、抗体発現、評価を含めた幅広い開発要件評価パッケージを提供しています。保有のクロマトグラフィー、分光光度計、蛍光測定機器群を使いて、開発工程、製剤化工程を見据えた最適な抗体候補を選ぶための柔軟な評価プログラムを提供します。

柔軟な開発可能性評価パッケージ
データ
開発要件評価のための標準物質
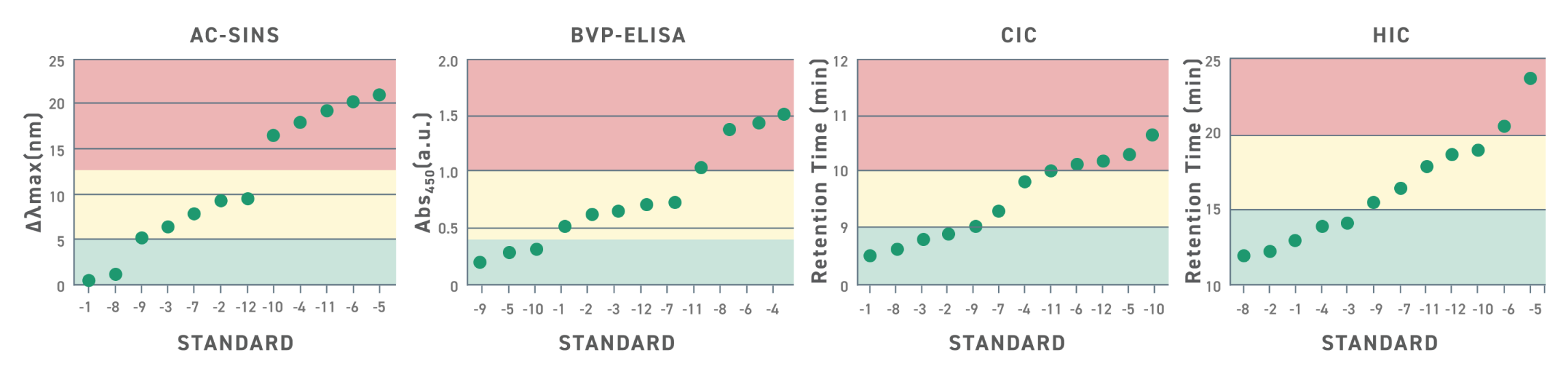
上の図は、12種類の標準物質を4種類の試験項目で評価した結果を示しています。それぞれの試験は候補抗体の相互作用や挙動に関する重要なデータを提供し、最も開発要件を満たす抗体配列を選ぶことを可能にします。各グラフ内の色分けされた領域は、下流の開発工程における許容範囲を示しています。
開発要件評価のための標準物質

上の図は、12種類の標準物質を4種類の試験項目で評価した結果を示しています。それぞれの試験は候補抗体の相互作用や挙動に関する重要なデータを提供し、最も開発要件を満たす抗体配列を選ぶことを可能にします。各グラフ内の色分けされた領域は、下流の開発工程における許容範囲を示しています。
技術資料
技術資料
技術資料
お問い合わせフォーム
抗体の専門家がお客様の開発ニーズについて詳しくお伺いいたします。難易度が高いと考えられる場合でもご遠慮なくお問い合わせください。