12. Dezember 2017
3 Min. Lesezeit
Aspirin, The 5000 Year-Old Wonder Drug
Aspirin has many uses, from fever and pain relief to prevention of cardiovascular disease and cancer.
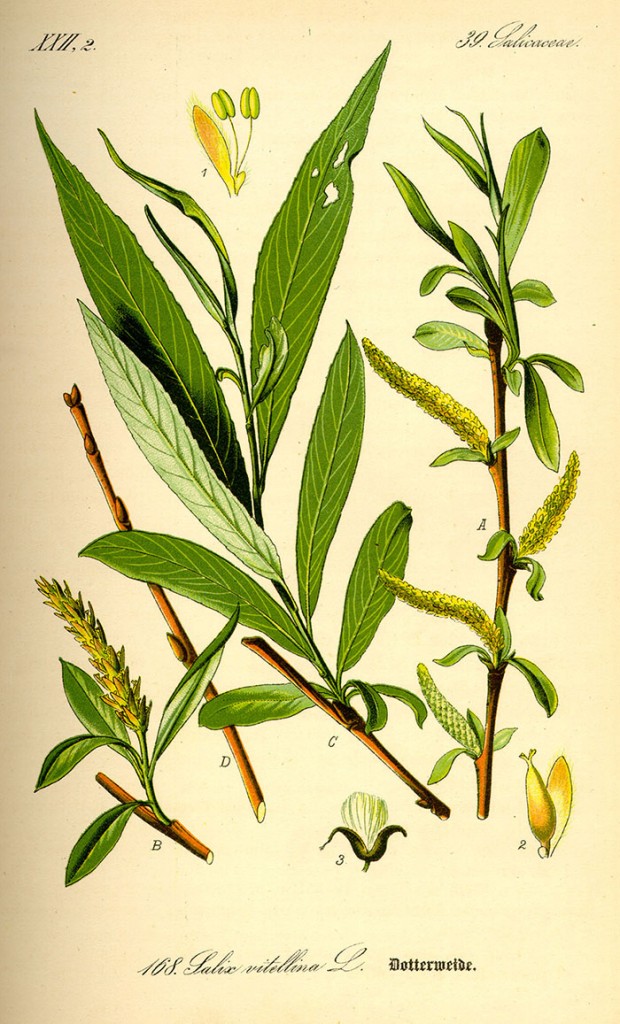
Aspirin has many uses today, from fever and pain relief to prevention of cardiovascular disease and cancer. It’s so ubiquitous that we often forget the long and arduous road that this drug has traveled to become a modern miracle. What we know as aspirin is the end result of efforts to alleviate pain that began with the use of willow as a medicine by the Sumerians and Egyptians in 3000 BC. The active ingredient in willow teas was eventually isolated, synthesized and sold as aspirin by Bayer at the beginning of the 20th century.
By 400 BC, willow leaf tea was in use by the Greeks to reduce the pain of childbirth, but the medicinal properties of willow weren’t re-discoveredin Europe until the 18th century, when a clergyman showed that powdered willow bark relieved patients with ague (fever). The active ingredient was isolated early in the 19th century from willow by Joseph Buchner at Munich University. He named the bitter, yellow crystals salicin. The first modern rigorous clinical trial of salicin was conducted 140 years ago, showing its utility for reducing fever and joint inflammation in rheumatism patients.
Salicylic acid was subsequently prepared from salicin by chemical means and used as a pain reliever. Unfortunately, it induced nausea and vomiting in large doses, and some patients even went into a coma. The drug needed to be “buffered” to eliminate its harmful effects on the stomach.
This buffering process converted salicylic acid into acetylsalicylic acid, the modern form of aspirin. This process was developed by German scientist Felix Hoffmann at Bayer pharmaceutical company. Bayer gave the drug the “aspirin” name and patented the buffering process. Aspirin’s journey from folk remedy to widely-used drug took over 4800 years.
You might think the rest is history. After all, aspirin is well-known, available at reasonable cost, and used all over the world. However, aspirin’s development saga continues even today, with as many as 700 to 1000 ongoing clinical trials every year. For example, the Women’s Health Study (WHS) revealed in 2005 that aspirin lowers the risk of stroke. Subsequent results from the same study in 2013 showed a 20% reduction in incidence of colon cancer in women with long-term use of low-dose aspirin. A separate study in 2011 revealed a 44% reduction in an inherited form of colon cancer in clinical trial participants who took aspirin for an average of four years.
The process is a little different now, of course, but drug development is still a long, difficult and costly process. Regulatory oversight of drug development has increased dramatically since the introduction of aspirin in the late 19th century. New drugs undergo extensive testing to clearly establish their safety and efficacy. Modern drugs are also often more complex and more difficult to produce than aspirin, further increasing the time to market. On average, it takes 12 years for a drug to go from the laboratory to the market In the United States, with an average cost estimated at almost $2.6 billion.
Developing the methods for designing, producing and purifying drugs can indeed be very time consuming. However, the new field of synthetic biology offers the potential to accelerate these steps. Using synthetic biology, organisms such as bacteria and yeast can be modified to produce small molecule drugs like aspirin, as well as “biological” drugs such as antibodies. The production process can also be rapidly improved, using synthetic biology tools and the resulting synthetic DNA to optimize the efficacy of these drugs and produce them in high yield and purity. Drug development is entering a new era that may bring more effective medicines to market—faster, and at lower cost—thanks to synthetic biology.
Was denken Sie?
Gefällt mir
Gefällt mir nicht
Gefällt mir sehr
Überraschend
Interessant
Nächsten Beitrag lesen
Blog abonnieren und die neuesten Informationen erhalten