Llamas are the unofficial mascots of Twist Bioscience. In addition to being a petting zoo favorite, they also generate antibodies that can be developed into drug candidates for cancers, autoimmune diseases and viral diseases.
We had llamas visit our South San Francisco location to bring our team together to learn more about llamas and remind us of one of the key tools our biopharma team uses to discover novel antibodies – synthetic libraries of camelid (a biological family that included llamas and alpacas) antibody sequences.
In addition to our South San Francisco team, our colleagues in Boston also rely on camelids to discover antibodies. Twist Boston developed and optimized an in vivo method for VHH (an antibody produced by camelids) discovery, which enables rapid identification of VHH antibodies that bind to biological targets on the cell surface and helps to maximize the chance of success of developing these antibodies into therapeutics or diagnostic reagents.

Single vs. Double Chain Antibodies
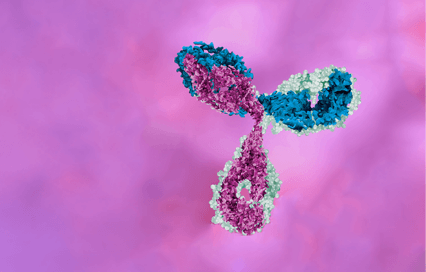
When thinking of an antibody, most people will likely picture a conventional immunoglobulin G (IgG) antibody (left, below). IgG antibodies have two chains, a heavy chain and a light chain. When these two chains come together the variable domains VH (located on the heavy chain) and VL (located on the light chain) form complementary determining regions (CDRs) where the antibody binds to targets, e.g. proteins, carbohydrates, and small molecules.
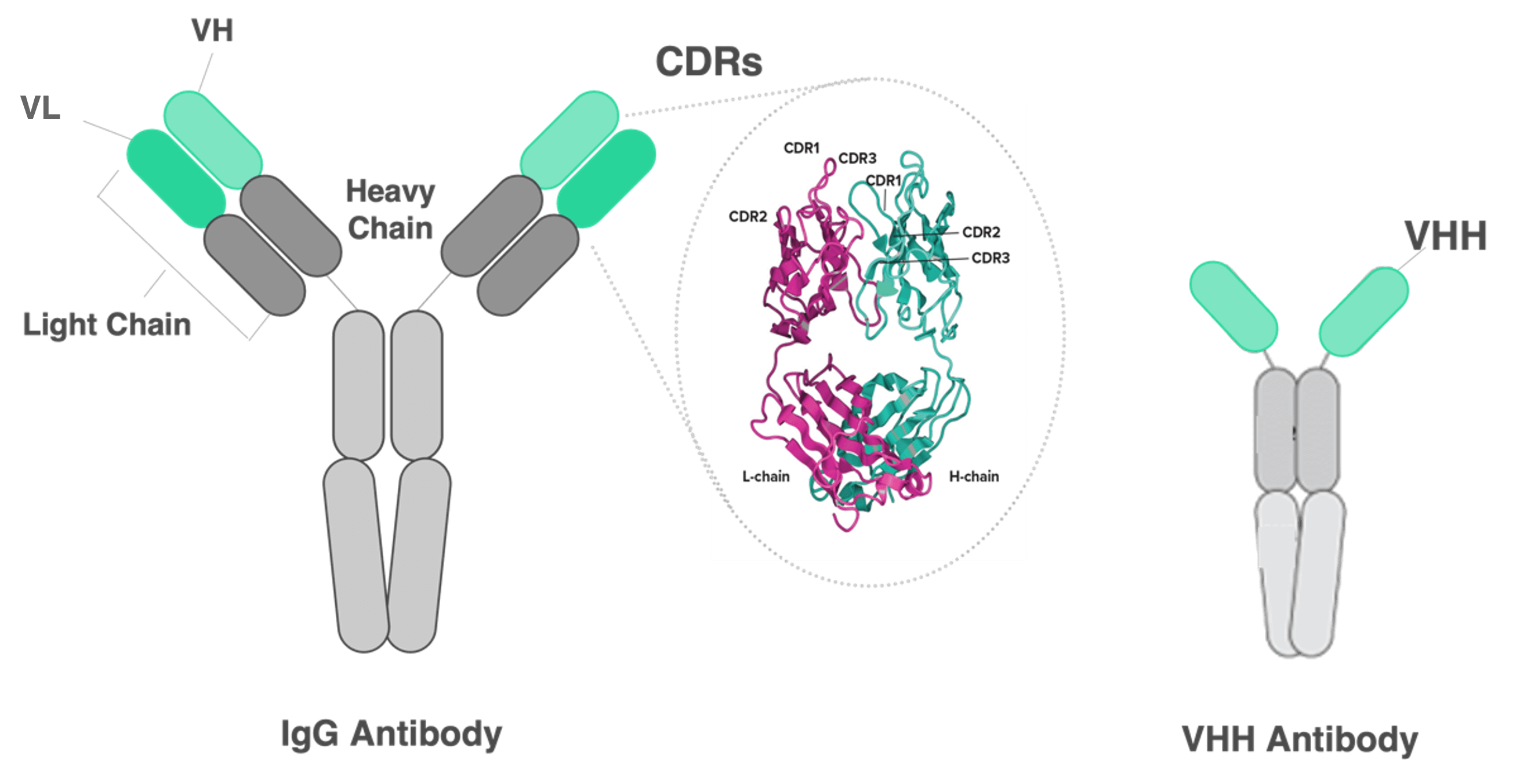
Camelids produce VHH antibodies (right, above), also called single domain antibodies, which have only one heavy chain with the variable domain VHH. VHH antibodies have structural differences compared to their IgG counterparts, making them more stable and allowing them to bind to targets with high affinity. They are also smaller, which allows them to bind to targets that are difficult for the bulkier IgG antibodies (like GCPRs associated with diseases such as cancer, which have small exposed surfaces with deep grooves).
While VHH antibodies can be developed on their own as therapeutic candidates, their modular nature makes them easily used to create bi- and multi-specific antibodies, which are ideal for developing next generation therapies for cancers, autoimmune diseases and viral diseases.
Creating VHH Libraries
The Twist Biopharma team has developed a suite of VHH libraries, each containing up to 10 billion antibodies, within the Library of Libraries. Twist uses novel methods that combine synthetic and natural approaches to maximize antibody diversity create VHH libraries that can be used against any protein target.
The VHH libraries include:
- VHH Ratio – synthetic oligo pools designed to model the natural VHH repertoire
- VHH Shuffle – natural llama CDR sequences shuffled within the context of a llama consensus framework
- VHH hShuffle – natural llama CDR sequences shuffled within the context of a partially humanized VHH framework
- VHH hShuffle HI - natural llama CDR1/2 shuffled with millions of human HCDR3 sequences within the context of a partially humanized VHH framework
- VHH hShuffle GPCR - natural llama CDR1/2 shuffled with nearly 3 billion GPCR -binding motifs in the hCDR3 within the context of a partially humanized VHH framework
Because of their ability to bind to difficult-to-reach targets and their high binding affinity, VHH antibodies have tremendous potential to be developed into drug candidates on their own or as a versatile building block in bi- and multi-specific antibodies for diseases such as cancers, autoimmune diseases and viral diseases.
Was denken Sie?
Gefällt mir
Gefällt mir nicht
Gefällt mir sehr
Überraschend
Interessant