What's The Key To ctDNA Detection? Good Preparation

Small fragments of DNA can tell important stories, particularly when they’re born from cancerous cells. Mutations carried through the bloodstream on wayward bits of DNA may betray the presence of a nascent tumor, one growing just beyond conventional detection limits. Still other mutations found in cell free DNA can tell of a tumor that is evolving therapeutic resistance. Naturally, circulating tumor DNA (ctDNA) and the stories it holds has become a promising source of clinical biomarkers, with burgeoning applications in early cancer detection, treatment guidance, and minimal residual disease testing.1-3
However, the elusive nature of ctDNA makes it exceedingly difficult to access. Blood plasma, urine, and other bodily fluids that are used for liquid biopsies are awash with cell free DNA (cfDNA) that’s been released from healthy tissues throughout the body. Within this genetic milieu, only about 0,01% to 10% of the total cfDNA fragments are likely to be recognized as tumor derived.4,5 Additionally, it is not always clear what the ctDNA will look like: tumor-associated mutations can occur in thousands of potential loci.
The value and potential of liquid biopsies is a topic of ongoing research. To reliably detect ctDNA, researchers thus need the ability to both limit the scope of sequencing to rare tumor-derived fragments—lest they waste a substantial amount of sequencing resources on irrelevant cell free DNA fragments—while also casting a broad net to ensure that all potential mutations are accounted for.5 Fortunately, recent advances in next-generation sequencing technology have helped improve the sensitivity and specificity of ctDNA detection, enabling optimization of both library preparation and target enrichment. Twist Bioscience has a library preparation kit (the Twist cfDNA Library Preparation Kit) that is designed specifically to support ctDNA research.
The Value of ctDNA
Circulating fragments of tumor DNA are increasingly viewed as valuable sources of clinical information, with potential applications in early cancer screening, minimal residual disease (MRD) testing, tumor profiling, and therapeutic decision making.1-3
"By its mere presence, ctDNA can be informative"
By its mere presence, ctDNA can be informative, indicating the potential existence of a malignant cell. Theoretically, ctDNA may be released from a single cancerous cell, opening the possibility of detecting malignant growths in their earliest stages. While there remains significant challenges to developing such a sensitive assay, several clinical applications of ctDNA are built around this principle. For example, ctDNA is proving to be a highly sensitive biomarker for minimal residual disease testing.1,6 Several studies have shown that ctDNA can be predictive of tumor recurrence, enabling the detection of recurrent tumors months before they become detectable by conventional means (in some cases, ctDNA identified tumors that never reached clinical detection thresholds).1,2,7,8 The success of these assays raises the real possibility of routinely using ctDNA for MRD and early cancer screening.
Equally important in all of this is the absence of ctDNA. Following curative treatments, many patients are proactively given adjuvant therapy in an effort to prevent tumor recurrence. However, these therapies are harsh in their own way and may cause harm. It’s been found that a negative MRD test correlates with a decreased likelihood of tumor recurrence and less of a need for adjuvant therapy. Therefore a true-negative can be used to spare patients from undue harm.9,10
Additionally, ctDNA naturally brings with it information about its malignant origin in the form of mutations and epigenetic signatures. The former can be used to build mutational profiles that expose the tumor’s therapeutic vulnerabilities (or lack-there-of).4,11 With the latter, methylation patterns may be used to determine what tissue the ctDNA was released from. This information can be helpful when detecting metastatic or nascent tumors.
To further develop and refine these potential applications, significant research is needed, as well as advances in the tools used to do it.
Preparing for Impact
The rarity of ctDNA presents substantial challenges to its detection.5 High quality target capture panels can be used to effectively enrich samples for ctDNA fragments, enabling deeper (and therefore higher confidence) sequencing. However, even the best target enrichment workflow can be foiled by poor library preparation.
Library preparation is the critical step in any sequencing workflow where targeted genetic material (in this case ctDNA) is made suitable for next-generation sequencing (NGS). This involves repairing single-strand overhangs on DNA fragments, ligating unique molecular identifiers and NGS adaptors to each end, and subsequent PCR amplification. Throughout this process, there are several opportunities for errors to occur which can substantially decrease the assay’s sensitivity and specificity. Two major sources of error are:
- Poor Conversion: Imperfect end-repair and ligation inefficiencies reduce the number of fragments that are properly converted over to a sequence-ready format. This is the most significant source of error and can greatly reduce assay sensitivity.5 Failed conversion can potentially eliminate the few ctDNA fragments that are present and lead to a false negative result.
- PCR Errors: Polymerases make mistakes roughly twice for every hundred-thousand base pairs amplified.12 When these errors occur early in the PCR process, the subsequent amplification of the error can make it appear to be legitimate, leading to a false-positive result.
Twist has developed a library preparation kit (the Twist cfDNA Library Preparation Kit) that is designed to minimize errors, maximize conversion rate, and enable confident ctDNA analysis. This is achieved through several optimizations, including carefully tuned end-repair and adaptor ligation reactions, use of a high-fidelity polymerase, and enabling the workflow to be compatible with duplex sequencing.
🧬 What is duplex sequencing?
Duplex sequencing is an elegant way to minimize false positives from polymerase and sequencing errors. The main goal of duplex sequencing is to use reads from both strands of a complementary DNA sequence to validate findings. During bioinformatic analysis, reads from both strands of the original source molecule are utilized to error-correct any false positives. When there is discordance between two complementary strands, it can be inferred that an error has occurred, and the observed mutation is artificial.
In comparison to other NGS library preparation kits recommended for cfDNA research (Figure 1), Twist’s approach leads to increased library conversion rate (ultimately measured as library yield) and an overall increased library complexity. When studying liquid biopsy samples, where the number of ctDNA fragments may be fewer than 10, having a high conversion rate is critical to assay sensitivity.
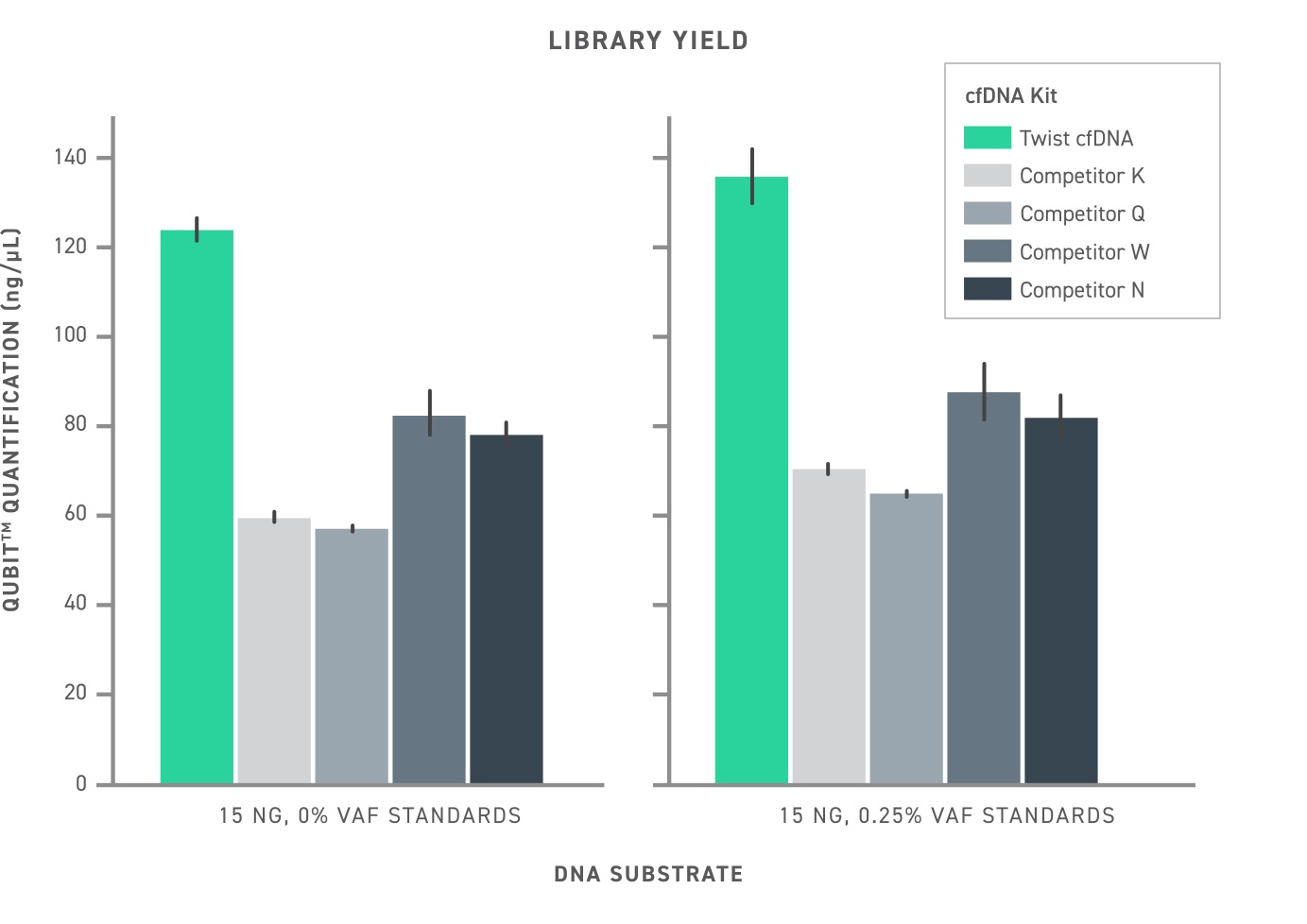
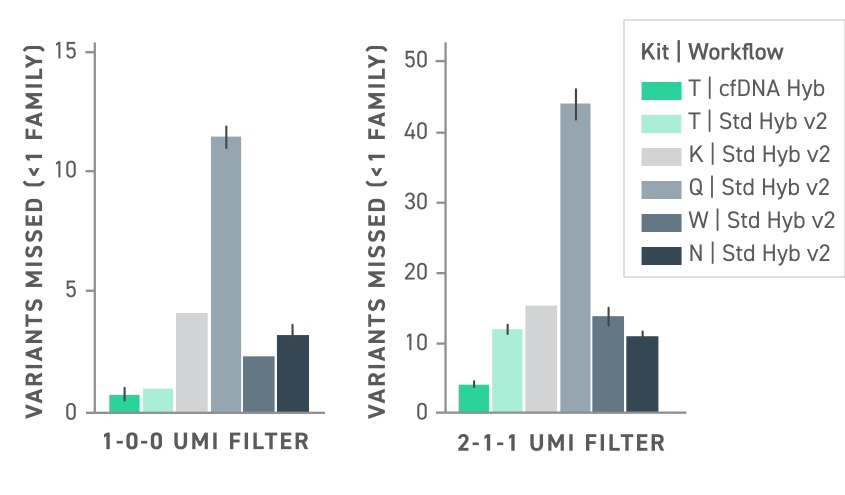
This is emphasized when attempting to detect low-frequency variants in cell free DNA (Figure 2). Whether using duplex sequencing or not, the Twist cfDNA Library Preparation Kit outperforms competitors by a wide margin, demonstrating very few missed variants.
Twist’s optimized library preparation workflow (particularly when paired with a Twist hybridization capture panel) thus empowers researchers to overcome some of the key challenges that have hindered ctDNA research. In this way, Twist is unblocking the field, enabling the development of ever more sensitive ctDNA assays for an expanding range of applications.
To learn more about the Twist cfDNA Library Preparation Kit, click here >>
Referencias bibliográficas
- Bronkhorst, Abel Jacobus, et al. “The Emerging Role of Cell-Free DNA as a Molecular Marker for Cancer Management.” Biomolecular Detection and Quantification, vol. 17, Mar. 2019, p. 100087, https://doi.org/10.1016/j.bdq.2019.100087.
- Abbosh, Christopher, et al. “Phylogenetic CtDNA Analysis Depicts Early-Stage Lung Cancer Evolution.” Nature, vol. 545, no. 7655, 26 Apr. 2017, pp. 446–451, www.nature.com/articles/nature22364, https://doi.org/10.1038/nature22364.
- Peng, Yan, et al. “Circulating Tumor DNA and Minimal Residual Disease (MRD) in Solid Tumors: Current Horizons and Future Perspectives.” Frontiers in Oncology, vol. 11, 18 Nov. 2021, www.ncbi.nlm.nih.gov/pmc/articles/PMC8637327/, https://doi.org/10.3389/fonc.2021.763790.
- Wan, Jonathan C. M., et al. “Liquid Biopsies Come of Age: Towards Implementation of Circulating Tumour DNA.” Nature Reviews. Cancer, vol. 17, no. 4, 1 Apr. 2017, pp. 223–238, https://doi.org/10.1038/nrc.2017.7.
- Song, Ping, et al. “Limitations and Opportunities of Technologies for the Analysis of Cell-Free DNA in Cancer Diagnostics.” Nature Biomedical Engineering, vol. 6, no. 3, 1 Mar. 2022, pp. 232–245, pubmed.ncbi.nlm.nih.gov/35102279/, https://doi.org/10.1038/s41551-021-00837-3.
- Faulkner, Lucy G., et al. “The Utility of CtDNA in Detecting Minimal Residual Disease Following Curative Surgery in Colorectal Cancer: A Systematic Review and Meta-Analysis.” British Journal of Cancer, vol. 128, no. 2, 8 Nov. 2022, pp. 297–309, https://doi.org/10.1038/s41416-022-02017-9.
- Zoe June Assaf, et al. “A Longitudinal Circulating Tumor DNA-Based Model Associated with Survival in Metastatic Non-Small-Cell Lung Cancer.” Nature Medicine, vol. 29, no. 4, 16 Mar. 2023, pp. 859–868, www.ncbi.nlm.nih.gov/pmc/articles/PMC10115641/, https://doi.org/10.1038/s41591-023-02226-6.
- Chaudhuri, Aadel A, et al. “Early Detection of Molecular Residual Disease in Localized Lung Cancer by Circulating Tumor DNA Profiling.” Cancer Discovery, vol. 7, no. 12, 2017, pp. 1394–1403, www.ncbi.nlm.nih.gov/pubmed/28899864, https://doi.org/10.1158/2159-8290.CD-17-0716.
- Sanz-Garcia, Enrique, et al. “Monitoring and Adapting Cancer Treatment Using Circulating Tumor DNA Kinetics: Current Research, Opportunities, and Challenges.” Science Advances, vol. 8, no. 4, 28 Jan. 2022, https://doi.org/10.1126/sciadv.abi8618.
- Tie, Jeanne, et al. “Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer.” New England Journal of Medicine, vol. 386, no. 24, 16 June 2022, pp. 2261–2272, https://doi.org/10.1056/nejmoa2200075.
- Thress, Kenneth S, et al. “Acquired EGFR C797S Mutation Mediates Resistance to AZD9291 in Non–Small Cell Lung Cancer Harboring EGFR T790M.” Nature Medicine, vol. 21, no. 6, 4 may de 2015, pp. 560–562, www.ncbi.nlm.nih.gov/pmc/articles/PMC4771182/, https://doi.org/10.1038/nm.3854.
- McInerney, Peter, et al. “Error Rate Comparison during Polymerase Chain Reaction by DNA Polymerase.” Molecular Biology International, vol. 2014, 2014, pp. 1–8, https://doi.org/10.1155/2014/287430.
¿Qué piensa?
Me gusta
No me gusta
Me encanta
Me asombra
Me interesa
